PT-141
Known as Bremelanotide in clinical contexts, PT-141 is a highly modified synthetic derived from
alpha-melanocyte-stimulating hormone. It has been put under rigorous clinical trials with the
goal of exploring its treatment potential regarding hypoactive sexual desire disorder in both
males and females, as well as for acute hemorrhage. PT-141 acts as a stimulator for the
melanocortin-4 and melanocortin-1 receptors. Studies have indicated its capability to strengthen
sexual arousal, as well as to stimulate the immune system.
DISCLAIMER:
This PRODUCT IS INTENDED FOR RESEARCH PURPOSES ONLY. It is designed for in vitro
testing and laboratory experimentation exclusively. All the information provided on this website
is purely for educational purposes. Under the law, any form of bodily introduction of this product
into humans or animals is strictly prohibited. It is essential that only licensed and qualified
professionals handle this product. This product is not intended to be used as a drug, food, or
cosmetic. It must not be misbranded, misused, or mislabeled as such. Its purpose and usage
are solely confined to research and scientific investigation.
Description
What Is PT-141?
Being involved in IIb clinical trials designed to address female hypoactive sexual desire disorder
(HSDD), PT-141 has gained the nickname “female Viagra”. PT-141 predominantly binds to
melanocortin 4 receptor (MC-4R) and MC-1R. Back in 2009, this melanocortin peptide was
included in the research regarding potential treatment for acute hemorrhage. It’s worth noting
that PT-141 is derived from Melanotan 2 (MT-2), another synthetic melanocortin.
PT-141 Molecular Structure
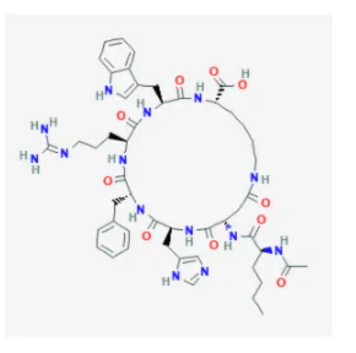
Source: PubChem
Sequence: Ac-Nle-Asp(1)-His-D-Phe-Arg-Trp-Lys(1)
Molecular Formula: C50H68N14O10
Molecular Weight: 1025.182 g/mol
PubChem CID: 9941379
CAS Number: 189691-06-3
PT-141 Research
PT-141 and Sexual Arousal
It is the only peptide known for stimulating the MC-4R, a receptor recognized for initiating
sexual arousal in the central nervous system, as well as having an effect on sexual behavior.
Research on mice has shown that binding to the said receptor through an agonist leads to
increased sexual arousal and heightened copulation in male and female subjects alike. It’s
important to mention that PT-141 operates differently compared to drugs like Viagra, suggesting
its potential as a treatment for sexual arousal disorders in both sexes, including those not
influenced by reduced blood flow to the genital area.
In a clinical trial with men experiencing erectile dysfunction (ED) who didn’t respond to Viagra
(sildenafil), subjects were administered Pt-141 via nasal spray. Approx. one third of the
participants successfully achieved erections sufficient for sexual intercourse. Results of the
study suggest a strong correlation between dosage and response, indicating the efficacy of
PT-141 in specific instances. These findings show the potential of PT-141 in regard to offering
insights into comprehending the main causes of hypoactive sexual desire disorder, while
plausibly offering treatment for erectile dysfunction when sildenafil doesn’t prove effective.
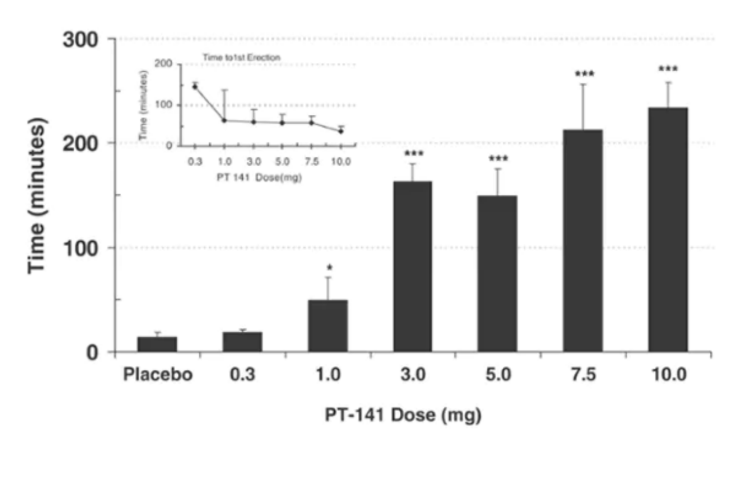
Duration of penile base rigidity greater than 60% for placebo compared to various doses of
PT-141.
Source: Nature
Prior to receiving approval, PT-141 was interestingly withdrawn from clinical trials. Peptide with
a potential for addressing women with hypoactive sexual desire disorder didn’t advance in
regards to treating said disorder, despite showing a notable rise in the frequency of satisfying
sexual events per month and a reduction in female sexual distress scores. This decision has left
many experts in the field of female sexual dysfunction disheartened, and they ascribed it to
the lack of established endpoints for FSD trials and societal biases against women’s sexual
health. Obstacles as such pose challenges to the approval process for much-needed treatments
like PT-141.
Said experts are advocating for raising awareness on the subject and establishing more specific
Food and Drug Administration (FDA) guidelines to assess therapies like PT-141 and its
benefits. They have said that a combination of therapies could potentially have synergistic
effects, and lament the absence of testing pharmacological treatments alongside already
established methods for addressing sexual dysfunction. These experts consider peptides like
PT-141 as beneficial not only for surmounting initial obstacles, but also for initiating
psychological treatment approaches.
In reaction to the outcry over the discontinuation of previous trials, Phase II Reconnect trials
were launched in 2017. The trials involve the use of subcutaneous injections of PT-141 to
address Female Sexual Dysfunction (FSD). Rekynda is the latest version of PT-141, and it is
anticipated to soon be accessible in the United States. This would authorize the off-label use of
PT-141 for treating sexual dysfunction in both men and women. The latest trials have introduced
modified endpoints, an appreciated development by the experts, since it increases the likelihood
of approval for such treatments.
PT-141 and Hemorrhage
Due to its strong binding affinity to MC-1R and MC-4R, PT-141 has shown the capability to
mitigate ischemia and protect tissues from inadequate blood supply during hypovolemic
(hemorrhagic) shock. This is why in 2009, PT-141 underwent minor adjustments while being
studied for its potential hemorrhagic shock treating capability. It’s worth mentioning that there
were minimal side effects when the drug was administered intravenously. The new, modified
form of PT-141 is now called PL-6983 and it has been assessed in phase IIb trials.
PT-141 and Infection
MC-1R exhibits anti-fungal and anti-inflammatory properties, as it has been observed when
studying a rat model with a particular fungal infection. Present anti-fungal treatments have
proven to be limiting in their mechanism of action, oftentimes leading to severe side effects in
certain individuals, hindering treatment, which is why this discovery proves significant.
Alternative methods of treating fungal infections have the potential of substantially lowering
morbidity and mortality rates, which can be accentuated in patients with compromised immune
systems.
PT-141 and Cancer
The MC-1R receptor holds a pivotal role in activating pathways for DNA repair, which makes it
noteworthy in regards to treating and preventing cancer. Research suggests that people with
varying forms of MC-1R could be more susceptible to basal cell and squamous cell carcinoma.
There is optimism regarding the potential of the modified PT-141 in addressing challenges
associated with these variants, providing potential pathways for preventing or treating these
specific cancer types.
Research Directions
At present, PT-141 is receiving significant attention in regards to treating sexual dysfunction.
The potential, however, goes beyond addressing sexuality and hemorrhage issues exclusively.
MC-4R, being a target of PT-141, is linked to specific instances of obesity, potentially
contributing to around 6% of cases of early-onset obesity. Studying PT-141’s influence on this
particular cause of obesity could reveal potential intervention pathways. Aside from MC-4R,
PT-141 also affects MC-1R, a receptor involved in pain, inflammation, kidney pathology and the
spread of infection, opening up diverse avenues for research.
When administered subcutaneously in mice, PT-141 demonstrates excellent bioavailability and
minimal side effects. It is of the utmost importance to adjust dosage considerations from mice to
humans. PT-141 offered at Peptide Sciences is exclusively meant for educational and scientific
research purposes. It is not appropriate for human consumption. Only licensed researchers
should make purchases.
Article Author
The above literature was researched, edited and organized by Dr. Logan, M.D. Dr. Logan holds
a doctorate degree from Case Western Reserve University School of Medicine and a B.S. in
molecular biology.
Scientific Journal Author
Dr. Sheryl A. Kingsberg is the chief of behavioral medicine at University Hospitals Case Medical
Center and professor in Reproductive Biology and Psychiatry at Case Western Reserve
University. Her areas of clinical specialization include sexual medicine, female sexual disorders,
cognitive behavioral psychotherapy, menopause, pregnancy and postpartum mood disorders,
psychological aspects of infertility, and psychological and sexual aspects of cancer. Dr.
Kingsberg’s primary research interests are in treatments for female sexual disorders and the
psychological aspects of infertility and menopause.
She led a randomized, placebo-controlled dose-finding trial for PT-141. She has numerous
publications in many national and international journals, sits on the editorial board of
Menopause and has authored numerous chapters on topics including perimenopause and
sexuality, oocyte donation, infertility and aging, the treatment of psychogenic erectile dysfunction
and sexuality after cancer.
Dr. Kingsberg received her PhD from the University of South Florida in Tampa and completed
her fellowship in sexual medicine at University Hospitals Case Medical Center. She is an active
member in a number of national and international organizations including the American
Psychological Association and the American Society for Reproductive Medicine. She currently
sits on the Board of Trustees of The North American Menopause Society, and serves as the
current treasurer of the Society for Assisted Reproductive Technologies. Dr. Kingsberg s a past
president of The International Society for the Study of Women’s Sexual Health.
Dr. Sheryl A. Kingsberg is being referenced as one of the leading scientists involved in the
research and development of PT-141. In no way is this doctor/scientist endorsing or advocating
the purchase, sale, or use of this product for any reason. There is no affiliation or relationship,
implied or otherwise, between Peptide Sciences and this doctor. The purpose of citing the
doctor is to acknowledge, recognize, and credit the exhaustive research and development
efforts conducted by the scientists studying this peptide. Dr. Kingsberg is listed in [12] under the
referenced citations.
Referenced Citations
- M. Sandrock, A. Schulz, C. Merkwitz, T. Schöneberg, K. Spanel-Borowski, and A.
Ricken, “Reduction in corpora lutea number in obese melanocortin-4-receptor-deficient
mice,” Reprod. Biol. Endocrinol. RBE, vol. 7, p. 24, Mar. 2009. - R. C. Rosen, L. E. Diamond, D. C. Earle, A. M. Shadiack, and P. B. Molinoff, “Evaluation
of the safety, pharmacokinetics and pharmacodynamic effects of subcutaneously
administered PT-141, a melanocortin receptor agonist, in healthy male subjects and in
patients with an inadequate response to Viagra,” Int. J. Impot. Res., vol. 16, no. 2, pp.
135–142, Apr. 2004. [PubMed] - H. Wessells, V. J. Hruby, J. Hackett, G. Han, P. Balse-Srinivasan, and T. W. Vanderah,
“Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2 induces penile erection via brain and spinal
melanocortin receptors,” Neuroscience, vol. 118, no. 3, pp. 755–762, 2003. [PubMed] - A.-S. Rössler, J. G. Pfaus, H. K. Kia, J. Bernabé, L. Alexandre, and F. Giuliano, “The
melanocortin agonist, melanotan II, enhances proceptive sexual behaviors in the female
rat,” Pharmacol. Biochem. Behav., vol. 85, no. 3, pp. 514–521, Nov. 2006. [PubMed] - M. R. Safarinejad and S. Y. Hosseini, “Salvage of sildenafil failures with bremelanotide: a
randomized, double-blind, placebo controlled study,” J. Urol., vol. 179, no. 3, pp.
1066–1071, Mar. 2008. [PubMed] - A. H. Clayton et al., “Bremelanotide for female sexual dysfunctions in premenopausal
women: a randomized, placebo-controlled dose-finding trial,” Womens Health Lond.
Engl., vol. 12, no. 3, pp. 325–337, 2016. [PubMed] - M. K. Miller, J. R. Smith, J. J. Norman, and A. H. Clayton, “Expert opinion on existing and
developing drugs to treat female sexual dysfunction,” Expert Opin. Emerg. Drugs, vol.
23, no. 3, pp. 223–230, 2018. [PubMed] - “AMAG Pharmaceuticals and Palatin Technologies Enter Into Exclusive Licensing
Agreement for North American Rights to RekyndaTM (bremelanotide), a Potential
Treatment for a Common Female Sexual Disorder – AMAG Pharmaceuticals.” .
[MarketWatch] - H. Ji et al., “The Synthetic Melanocortin (CKPV)2 Exerts Anti-Fungal and
Anti-Inflammatory Effects against Candida albicans Vaginitis via Inducing Macrophage
M2 Polarization,” PLoS ONE, vol. 8, no. 2, Feb. 2013. [PLOS ONE] - V. Maresca, E. Flori, and M. Picardo, “Skin phototype: a new perspective,” Pigment Cell
Melanoma Res., vol. 28, no. 4, pp. 378–389, Jul. 2015. [PubMed] - L. Feller, R. a. G. Khammissa, B. Kramer, M. Altini, and J. Lemmer, “Basal cell
carcinoma, squamous cell carcinoma and melanoma of the head and face,” Head Face
Med., vol. 12, p. 11, Feb. 2016. [PubMed] - Clayton AH, Althof SE, Kingsberg S, et al. Bremelanotide for female sexual dysfunctions
in premenopausal women: a randomized, placebo-controlled dose-finding trial. Womens
Health (Lond). 2016;12(3):325–337. doi:10.2217/whe-2016-0018 - T. R. McMillan, M. A. M. Forster, L. I. Short, A. P. Rudecki, D. L. Cline, and S. L. Gray,
“Melanotan II, a melanocortin agonist, partially rescues the impaired thermogenic
capacity of pituitary adenylate cyclase-activating polypeptide deficient mice,Exp. Physiol.
, vol. 106, no. 2, pp. 427–437, Feb. 2021, doi: 10.1113/EP088838.” - C. Spana, R. Jordan, and S. Fischkoff, “Effect of bremelanotide on body weight of obese
women: Data from two phase 1 randomized controlled trials,Diabetes Obes. Metab., vol.
24, no. 6, pp. 1084–1093, Jun. 2022, doi: 10.1111/dom.14672. ”
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR
INFORMATONAL AND EDUCATIONAL PURPOSES ONLY.
The products offered on this website are furnished for in-vitro studies only. In-vitro studies (Latin:
in glass) are performed outside of the body. These products are not medicines or drugs and
have not been approved by the FDA to prevent, treat or cure any medical condition, ailment or
disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.